Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kaneko, Masashi; Sasaki, Yuji; Wada, Eriko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 50(10), p.1765 - 1769, 2021/10
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)Density functional theory calculation is applied to predict the stability constants for Eu and Am
and Am complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
 0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am
0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am selectivity over Eu
selectivity over Eu .
.
Tochiyama, Osamu*
JNC TJ8400 2000-044, 53 Pages, 2000/02
To estimate the polyelectrolyte effect and the effect of the heterogeneous composition of humic acids, the complex formation constants of Eu(III) and Ca(II) with Aldrich humic acid and polyacrylic acid were obtained, for Eu(10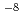 to 10
to 10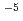 M) by solvent extraction with TTA and TBP in xylene, for Ca (10
M) by solvent extraction with TTA and TBP in xylene, for Ca (10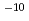 M) with TTA and TOPO in cyclohexane and for Ca(10
M) with TTA and TOPO in cyclohexane and for Ca(10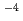 M) by using ion-selective electrode. By defining the apparent formation as
M) by using ion-selective electrode. By defining the apparent formation as 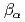 = [MR
= [MR ]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR
]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR ] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log
] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log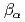 have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO
have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO and NaCl. Log
and NaCl. Log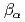 of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log
of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log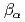 increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log
increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log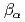 , the decrease in log
, the decrease in log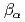 of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log
of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log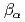 of polyacrylate, log
of polyacrylate, log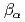 of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log
of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log 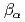 of humate by 0 to 0.8. The dependence of log
of humate by 0 to 0.8. The dependence of log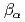 of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.
of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO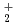 and AnO
and AnO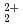 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO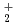 and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Lothenbach, B.*; Ochs, M.*; Wanner, H.*; Yui, Mikazu
JNC TN8400 99-011, 340 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of palladium Pd, lead Pb, tin Sn, antimony Sb, niobium Nb and bismuth Bi in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system of high-level radioactive wastes. Besides treating hydrolysis in detail, this report focuses on the formation of complexes or compounds with chloride, fluoride, carbonate, nitrate, sulfate and phosphate. Other important inorganic ligands (sulfide for lead and antimony, ammonia for palladium) are also included. In this study, the specific ion interaction theory (SIT) approach is used to extrapolate thermodynamic constants to zero ionic strength at 25 C.
C.